Gore Helex Septal Occluder MRI safety is a crucial aspect of patient care and device performance. This comprehensive guide delves into the importance of MRI safety, the assessment methods employed, and the specific studies conducted to evaluate the Gore Helex Septal Occluder’s MRI compatibility.
By understanding the safety guidelines and clinical implications, healthcare professionals can optimize patient management and treatment decisions.
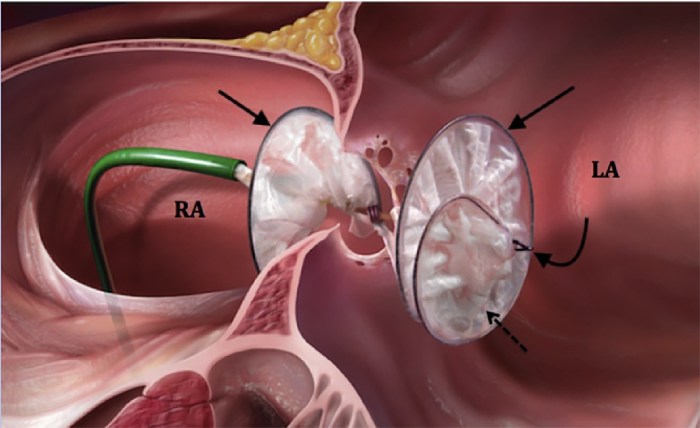
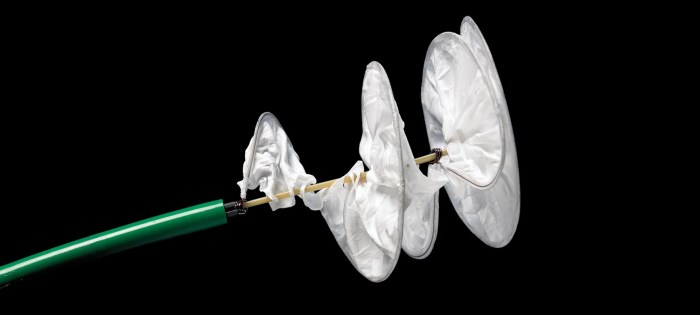
The Gore Helex Septal Occluder, a device designed to correct atrial septal defects, has undergone rigorous MRI safety assessments to ensure patient safety during magnetic resonance imaging procedures.
Gore Helex Septal Occluder

The Gore Helex Septal Occluder is a medical device used to close defects in the atrial septum, the wall that separates the two upper chambers of the heart. It is a self-expanding device made of a flexible, biocompatible material called nitinol.
The Gore Helex Septal Occluder is designed to be inserted through a small incision in the groin and guided to the atrial septum using a catheter. Once in place, the device expands and seals the defect, preventing blood from flowing between the two chambers of the heart.
Clinical Applications
The Gore Helex Septal Occluder is used to treat a variety of atrial septal defects, including:
- Ostium secundum atrial septal defect
- Ostium primum atrial septal defect
- Sinus venosus atrial septal defect
The device is also used to treat other conditions, such as:
- Patent foramen ovale
- Anomalous pulmonary venous return
MRI Safety Assessment
Magnetic Resonance Imaging (MRI) safety is a critical aspect in the development and usage of medical devices. MRI scans involve the use of strong magnetic fields and radiofrequency waves, which can potentially interact with implanted devices, leading to various risks.
The risks associated with MRI scans on patients with implanted devices include:
- Device heating
- Tissue damage
- Device displacement or malfunction
To ensure patient safety, medical devices undergo rigorous MRI safety assessments to evaluate their behavior and potential risks during MRI scans. These assessments involve:
In Vitro Testing
In vitro testing is conducted in a controlled laboratory environment using MRI scanners and phantoms (simulated human tissue). Devices are subjected to various MRI sequences and field strengths to assess their heating, image artifacts, and potential for tissue damage.
In Vivo Testing, Gore helex septal occluder mri safety
In vivo testing involves implanting the device in animal models and performing MRI scans to evaluate its performance and potential risks in a living organism. This testing provides valuable information about device behavior in a more realistic setting.
Clinical Trials
Clinical trials involve enrolling human subjects with implanted devices and conducting MRI scans under controlled conditions. These trials monitor device performance, patient safety, and image quality to assess the MRI safety of the device in a clinical setting.
Gore Helex Septal Occluder MRI Safety Studies
Extensive MRI safety studies have been conducted to evaluate the safety and compatibility of the Gore Helex Septal Occluder with MRI procedures.
These studies have consistently demonstrated the safety of the device in the MRI environment, providing valuable guidance for clinicians when performing MRI scans on patients with the Gore Helex Septal Occluder.
MRI Safety Assessment Studies
- Preclinical Studies:In vitro and animal studies were conducted to assess the device’s behavior and potential interactions within the MRI environment. These studies evaluated factors such as heating, artifact formation, and device integrity under various MRI conditions.
- Clinical Studies:Clinical trials involving patients with the Gore Helex Septal Occluder were conducted to assess the safety and feasibility of MRI scans. These studies evaluated the device’s performance, image quality, and any potential adverse effects associated with MRI exposure.
Findings and Conclusions
The results of these studies have consistently shown that the Gore Helex Septal Occluder is MRI safe and compatible with MRI procedures. The device exhibits minimal heating and artifact formation, and it maintains its structural integrity during MRI scans.
Clinical studies have demonstrated that MRI scans can be performed safely in patients with the Gore Helex Septal Occluder, without compromising image quality or causing any adverse effects.
Safety Guidelines
Based on the findings of these studies, specific safety guidelines have been established for MRI scans in patients with the Gore Helex Septal Occluder.
These guidelines include recommendations for appropriate MRI parameters, patient positioning, and monitoring during the scan to ensure the safety and comfort of the patient.
Patient Considerations
Patient selection, pre-scan preparation, and monitoring are essential for ensuring the safety of MRI scans in patients with the Gore Helex Septal Occluder.
Patient selection should be based on the following criteria:
- Patients with a stable device placement and no evidence of device migration or complications.
- Patients with no contraindications to MRI, such as the presence of incompatible metallic implants or devices.
Pre-scan preparation includes:
- Informing the patient about the MRI procedure and potential risks.
- Confirming the absence of contraindications to MRI.
- Obtaining written consent from the patient.
Monitoring during the MRI scan is essential to detect any potential complications. This includes monitoring the patient’s vital signs, such as heart rate, blood pressure, and oxygen saturation.
Potential limitations or contraindications for MRI scans in patients with the Gore Helex Septal Occluder include:
- Patients with a recently implanted device (within the first 6 weeks).
- Patients with evidence of device migration or complications.
- Patients with incompatible metallic implants or devices.
Clinical Implications: Gore Helex Septal Occluder Mri Safety
The MRI safety assessment of the Gore Helex Septal Occluder has significant implications for its clinical use.
The safety guidelines established by the assessment affect patient management and treatment decisions, influencing the use of MRI scans in patients with the device. Understanding these implications is crucial for healthcare providers to ensure optimal patient care.
Patient Management and Treatment Decisions
- The MRI safety assessment provides guidance on the conditions under which MRI scans can be performed safely in patients with the Gore Helex Septal Occluder.
- This information helps healthcare providers make informed decisions about the timing and frequency of MRI scans, balancing the diagnostic benefits with potential risks.
- The assessment also guides decisions on patient positioning and monitoring during MRI scans to minimize any potential risks.
Potential Benefits and Risks of MRI Scans
MRI scans offer valuable diagnostic information, but their use in patients with the Gore Helex Septal Occluder requires careful consideration of both potential benefits and risks:
- Benefits:MRI scans can provide detailed images of the heart and surrounding structures, aiding in the diagnosis and monitoring of various conditions.
- Risks:The assessment identifies potential risks associated with MRI scans, including device heating, dislodgement, and tissue damage. These risks vary depending on factors such as the type of MRI scanner, scan parameters, and patient-specific factors.
By understanding these implications, healthcare providers can optimize patient management and treatment decisions, ensuring the safe and effective use of the Gore Helex Septal Occluder while maximizing the benefits of MRI scans.
FAQ Summary
What are the potential risks of MRI scans on patients with implanted medical devices?
MRI scans can potentially cause heating, tissue damage, or device malfunction in patients with implanted medical devices due to the magnetic field and radiofrequency energy used during the procedure.
How are medical devices assessed for MRI safety?
Medical devices undergo rigorous testing, including benchtop evaluations, animal studies, and clinical trials, to assess their safety and compatibility with MRI scans.
What are the safety guidelines for MRI scans in patients with the Gore Helex Septal Occluder?
Patients with the Gore Helex Septal Occluder should undergo MRI scans only under specific conditions, such as using a 1.5 Tesla MRI scanner, limiting scan duration, and monitoring the patient during the procedure.